Neonatal Airway Stabilization System
-
Role: Technical Strategy / MBA cross-functional team
-
Scope: Supported commercialization evaluation for a patented NICU device
-
Focus: User research with clinicians, product feasibility, risk, and system-level strategy

Define
Premature and critically ill infants require intubation for effective ventilation, but maintaining secure placement of endotracheal tubes (ETTs) is a major clinical challenge:
-
~98% of premature infants are intubated; ~40% experience tube dislodgement.
-
Effective ventilation requires ±0.5 cm positional accuracy.
-
Current devices rely on adhesive tape and plastic supports that often fail under patient movement, moisture, and routine handling.
Clinical & System Consequences:
-
Repeated sedation and airway instability disrupt blood pressure and heart rate.
-
~33% adjustment failure rate leads to repeated taping and X-rays (~500 additional/year, ~$500k cost).
-
Workflow interruptions for nurses and respiratory therapists; increased medicolegal risk.

Goal
-
Evaluate the commercial potential of the LETT device, a patented neonatal ETT securement solution featuring the double-spring locking/adjustment mechanism
-
Provide actionable recommendations for next steps in licensing, commercialization, or early adoption.
Approach
Empathize & Understand Users
-
Conducted 20+ interviews with a mix of NICU nurses, respiratory therapists, pediatric surgeons, and market experts.
-
Documented real-world pain points, including frequent re-taping, workflow disruption, and fear of accidental extubation.
Technology Analysis
-
Deep dive into the LETT device mechanics, including the double-spring locking mechanism.
-
Assessed current prototype status, potential enhancements, and development readiness.
-
Identified key benefits: precise ETT adjustment without tube removal, reduced X-ray usage, stronger securement.
Competitive & Market Assessment
-
Full competitive analysis comparing LETT against incumbents like Neobar.
-
Evaluated strengths, weaknesses, opportunities, and threats (SWOT) across technical, commercial, and regulatory dimensions.
-
Performed market sizing:
-
Total addressable market: $2.21B globally
-
Serviceable addressable market (ETT securement): $160M
-
Serviceable obtainable market (neonatal segment): ~$16M
-
Business & Commercial Strategy
-
Developed a full business model canvas covering value proposition, customer segments, channels, revenue streams, and partnerships.
-
Analyzed two potential go-to-market strategies: licensing to existing medical device companies vs. direct manufacturing and sales.
Valuation & Recommendations
-
Created a detailed valuation model using:
-
Long-term savings NPV (~$5.42M)
-
Comparable company multiples (~$12M)
-
Qualitative scorecard (~$8.51M)
-
-
Weighted triangulated valuation: ~$7.66M
-
Recommended next steps: continue functional testing, validate efficacy, and pursue licensing opportunities to established device companies to reduce risk while leveraging market reach.




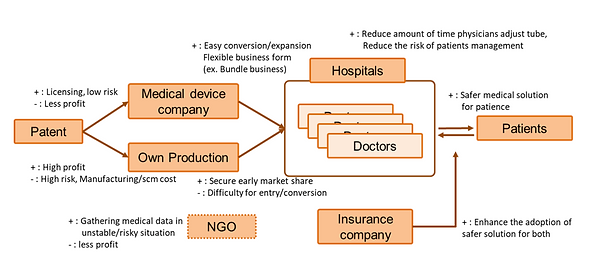
Impact
-
Delivered a comprehensive Technology Readiness Report summarizing market potential, competitive landscape, technology benefits, and commercialization pathways.
-
Identified actionable strategies to reduce workflow burden, patient risk, and operational costs in NICUs.
-
Provided a structured framework for go/no-go commercialization decisions, setting the stage for early adoption and strategic partnerships.

